Theory of Constraints, Critical Chain Project Management, Lean : industry consulting & training - Marris Consulting
How to get a portfolio of projects back on track in the medical devices industry
Context
Our client develops medical testing devices for laboratories and hospitals. They want to launch a new range of products as fast as possible since speed is a critical success factor in their industry.
But the project managers have changed many times, the teams are stressed, and the management team is increasing the pressure.
These are international projects, with teams in Spain, the UK, Germany, and the US.
But most projects are late.
That is the reason why our client called us for help.
Approach
To get the projects back on track, we implemented a 3-fold approach :
-
Strengthen execution, mainly documentation, which is heavy work in this industry. All the tasks and assays are documented, reviewed, and validated before submission to the regulatory administrations.
-
Reinforce project management. Insist on planning and follow-up to improve the coordination of the teams and properly size resources.
-
Streamline portfolio management to prioritize projects, align the strategy and tactics, and focus resources where they matter most.
Routines and tools
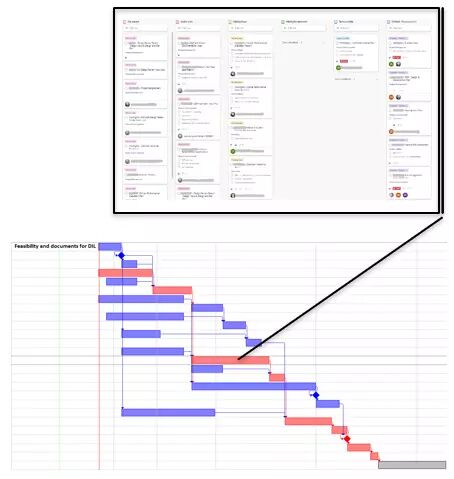
We started by listing all the documents with the teams to assess their progress and established a weekly review with them. An online Microsoft Planner kanban board was installed to track all the documents and identify the person in charge and the problems when they arise. This routine tremendously accelerated the documentation process.
One anecdote: everybody thought Quality Assurance was slowing down the documentation process, but the routine showed that many questions and related documents were waiting for management's and marketing's answers.
Critical Chain plans were built with the teams. They clearly showed that the verification and validation phase needed more instruments to go faster, as well as additional sites.
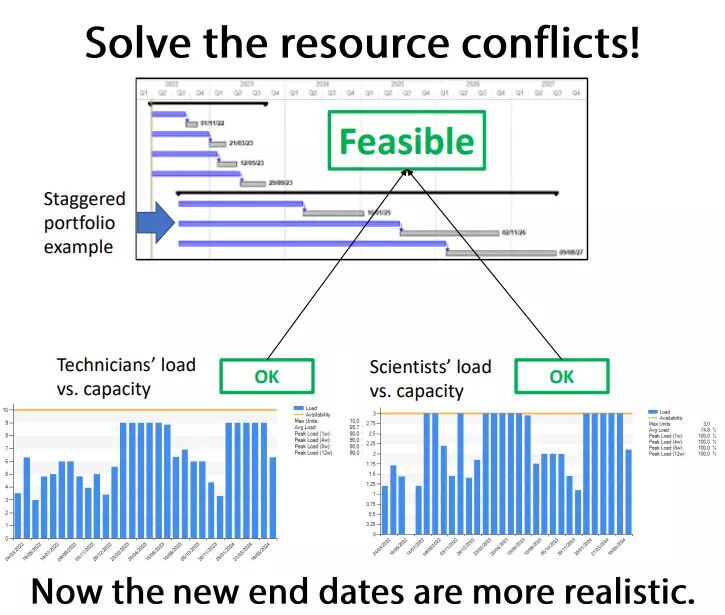
Eventually, multi-project planning sessions were organized to synchronize all the projects. Indeed, these projects required shared resources, which were previously spread thin on too many topics. After identifying the bottlenecks, some projects were staggered and rescoped to mitigate delay risks.
Results
Products are launched on the market faster than before, and delays are more predictable thanks to the Critical Chain approach and buffer management.
- Between 2 and 6 months saved on the two main projects.
- Respiratory products are finished on time or with minor delays.
- Risk mitigation of the most critical project's strategy and Portfolio Management.
About Marris Consulting
Marris Consulting is an industry consulting and training company specialized in the Theory of Constraints (ToC) and Critical Chain Project Management. We focus on improving the performance of manufacturing and process industries by using Constraints Management combined with Lean and Six Sigma. To boost project performance, we also use Critical Chain Project Management (CCPM), which we sometimes combine with Lean Engineering. Our 2-day performance audits, our performance consulting services and our project management, Lean, ToC & CCPM training by our industry consultants offer a wide range of solutions to help our clients around the world reach the highest possible levels of performance.


